What Does a Negative Reduction Potential Mean
A positive reduction potential means that the reduced form of a substance has higher affinity for electrons than does H 2. Reduction potential indicates the power to add hydrogen lose oxygen or attract electrons.
A negative reduction potential means that the reduced form of a substance has lower affinity for electrons than does H 2.
. Fluorine gas is one of the best oxidizing agents there are and it is at the top of the table with the biggest most positive standard potential 287 V. A positive ORP indicates the presence of potential oxidizers while a negative ORP indicates the presence of potential reducers. In general a decrease in reduction potential upon complex formation has been observed.
Answered 6 years ago Author has 11K answers and 1M answer views. A negative reduction potential tells us that the zinc ion is harder to reduce is a worse oxidizing agent than is the hydrogen ion. If the reduction potential is negative that molecule is more likely to give up electrons and become oxidized.
The energy of an electron in vacuum. ORP is measured in millivolts mVs using an ORP meter. A negative value of cell potential indicates a reducing environment while a.
If you study potential of electrons in atom you will find it to be negative. This means that the desired path of the reaction is actually the reverse reaction. Microbial-mediated redox processes can decrease the redox potential to a level as low as 300 mV.
A healthy ORP for the human body is between -150 and -400mV. A positive ORP means that the substance is an oxidizing agent and a negative ORP means the substance is a reducing agent. Negative ORP is in ionized water and fresh uncooked foods.
As the redox potential increases in value and turns positive its ability to oxidize is enhanced. The standard cell potential is the potential difference between the cathode and anode. The standard potentials are all measured at 298 K 1 atm and with 1 M solutions.
The positively charged nucleus attracts the negatively charged electrons. Positive ORP increases oxidation aging and is found in tap water bottled waters distilled and reverse osmosis waters as well as cooked and processed foods. It is the negative ORP that is beneficial to our body in that it reduces oxidation anti-oxidant.
What is the oxidation-reduction potential of water. The measurement is done using an ORP meter which includes. Oxidationreduction potential Eh is a measure of the ability of chemicalbiochemical systems to oxidize lose electrons or reduce gain electrons.
The ve sign of standard reduction potential indicates that the electrode when joined with SHE acts as cathode and reduction occurs on this electrode spontaneously. In reduced environments such as in the deep water of stratified lakes or the sediment of eutrophic lakes the redox potential will be low below 100 mV or even negative. It has some resemblance to pH value of a liquid.
If you see Earths potential it is negative as it is attractive. The energy of a bound electron is negative compared to the energy of an electron in vacuum. This change in reduction potential although not popular in synthetic coordination chemistry can be made as a basis for the detection of complex formation.
During reduction reaction oxidation state of the an element decreasesThe standard reduction value on an element is determined with respect to the reduction potential of hydrogenThe standard reduction potential of hydrogen is 00 VoltsWith negative standard reduction potential means that they have higher tendency to loose electronsBy loosing of. On the right side product side are substances that want. This also tells us that zinc metal is a.
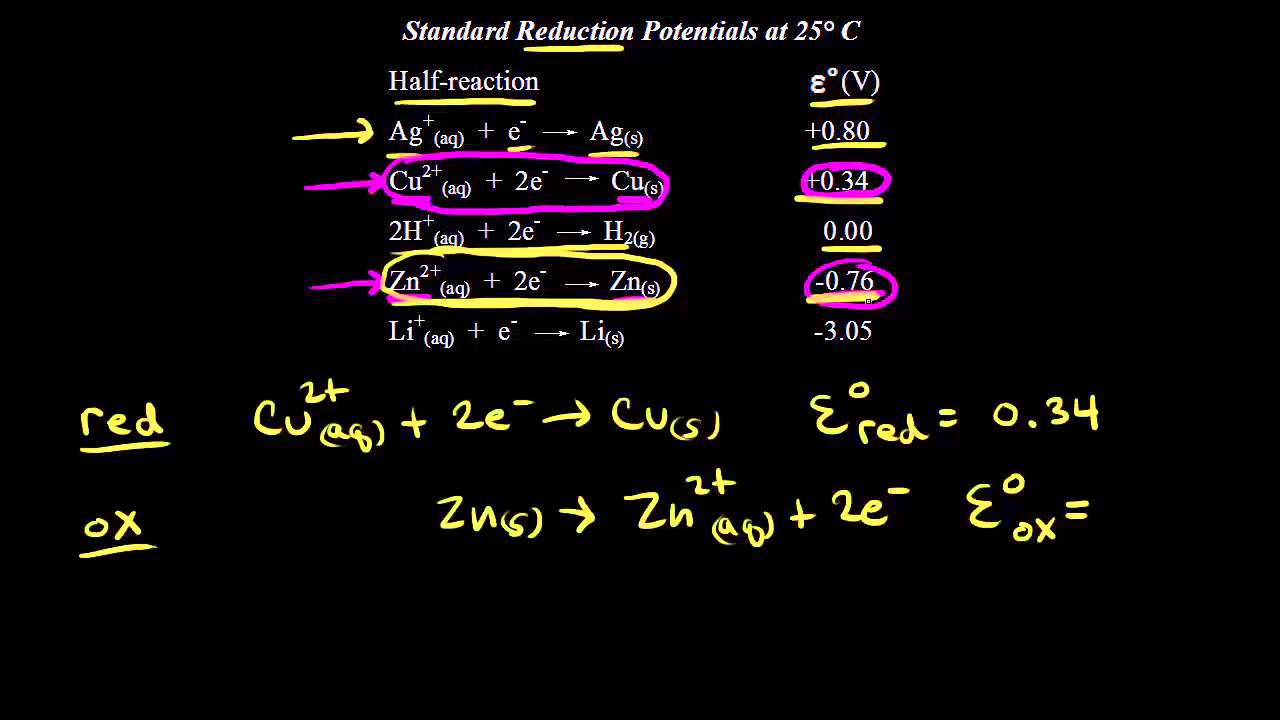
2e Zn 2 aq Zn s Note. The standard reduction potential is in a category known as the standard cell potentials or standard electrode potentials. Strong reducing agents have high standard reduction potentials in quantum ie the numbers are larger but these potentials are NEGATIVE so the smaller the number ie the further below zero it is the greater the reducing strength.
In situations where an electrochemical series is not sufficient to absolutely determine the direction of a redox reaction the standard electrode potential E o can be used. Positive sign of reduction potential physically signifies non-spontaneous reduction reaction and negative sign indicates spontaneous reduction reaction. This means that the higher oxidation states of the metal ion become more stable after complexation.
When it decreases in value and turns negative its reducing ability is quantitatively enhanced. ORP or oxidation-reduction potential also called redox potential is a measurement of waters tendency to act as either a reducing agent electron donor or oxidizing agent electron acceptor. Second the convention that a more negative reduction potential means greater reducing strength is useful because it goes with another important scale the energy of an electron vs.
The Eh of milk is about 150 mV and that of cheese is about -250 mV. At the other end are reactions with negative standard potentials. Negative potential is just a potential that has attractive nature.
The standard reduction potential for the zinc electrode is 076 V. A positive value indicates an oxidized state whereas a negative value indicates a reduced state. For more information view Cell Potentials.
Standard Reduction Potentials Video Khan Academy

Difference Between Reduction Potential And Reducing Power Compare The Difference Between Similar Terms
Comments
Post a Comment