Which Words Best Describe a Pure Sample of Glucose
Download the full version above. If the composition of a sample varies the sample is a pure substance.

In The Womb National Geographic Worksheet Answer Key Prior To Preaching About In The Womb National Geographic Worksheet Answer Key Remember To Be Aware That
There is 10 water inside the cell and 75 water outside the cell.
. D stands for dextro and L stands for levo. The test can be done at any time of the day. The student then uses a testing strip to indicate if glucose is present.
The result is best describe as. If a 827-g sample of glucose contains 0555 g of hydrogen. If the color of the result remains blue.
A chemical property of oxygen. Which of the following does NOT describe a pure sample of glucose C6H12O6. What is the best method to purify salt water.
6 CS 2020 06203320 3 Some properties of four substances A B C and D are shown in the table. If the OH is on the right it is a D sugar in this case D-glucose. The second statement is false because although glucose is made up of three types of atoms it is not an element.
Elements and compounds are pure substances. Renal threshold of glucose THRESH-hold The blood sugar blood glucose concentration at which the kidneys start to excrete glucose into the urine. It is found in fruits and honey and is the major free sugar circulating in.
When written in this particular way the position of the OH on the last asymmetric carbon atom will tell us whether we are dealing with a D sugar or an L sugar. A pure substance has a fixed composition which you can see glucose has from the chemical chemical formula. The benedict solution is a type of solution which can identify the presence of sugar on a sample of substances.
If glucose is not present the testing strip will stay white. If the composition of a sample is fixed the sample is a. A The water sample from the stream would have more oxygen because that sample has been open to the air.
When you have diabetes your body isnt able to get the sugar from blood into cells or make enough or any insulin. Bitter-sweet tasting bitter and sweet at the same time. C6H12O6 which is glucose is acompound and therefore a pure substance.
Glucose also called dextrose one of a group of carbohydrates known as simple sugars monosaccharides. A pure substance or chemical substance is a material that has a constant composition is homogeneous and has consistent properties throughout the sample. This causes high levels of.
For the food sample if the glucose or reducing sugars would be present the color will change. A cell has 90 NaCl on the inside and 75 water on the outside. On a scale or balance.
In the presence of glucose the solution turns a red color reports The Science Company. Pure substances A pure substance has a definite and constant composition like salt or sugar. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary.
Elements An element is composed of a single kind of atom. Yellow or brick red. The water will move inside the cell.
Select all the statements that correctly describe mass andor weight. In chemistry a pure substance consists of only one type of atom molecule or compound. Oxygen will support combustion.
All particles in a sample of a pure substance are the same. Glucose is transported from the intestines or liver to body cells via the bloodstream and is made available for cell absorption via the hormone insulin produced by the body primarily in the pancreas. Retinopathy REH-tih-NOP-uh-thee Eye disease that is caused by damage to the small blood vessels in the retina.
Bitter a strong sharp taste that is not sweet. Describe a test to show that a food sample contains glucose - 15361902. The first statement is incorrect because glucose is a molecule and a molecule cant contain molecules.
This page of the essay has 4408 words. Glucose is the primary source of energy for the bodys cells and blood lipids in the form of fats and oils are primarily a compact energy store. Astringent an astringent taste is one that is strong and bitter.
It can contain atoms that can make up other molecules but it cant actually contain other molecules. Distillation chromatography fractional. To identify whether reducing sugar or non-reducing sugar is presence on the chosen samples which are glucose hydrolyzed sucrose non-hydrolyzed sugar starch and water.
C The bottled-water would give the best results because that water was purchased. The science team is just drooling over these picturesThe Hubble. The glucose challenge test is the short version of the glucose tolerance test.
This test exposes glucose to a clear blue solution of sodium and copper salts. Brackish has a slight taste of salt and is therefore not pure. In every mammal Glucose is acts as an important energy source metabolic substrate.
It involves drinking a glass of concentrated glucose solution 50 g of glucose dissolved in 250 to 300 ml of water. After one hour has passed a blood sample is taken to determine the blood sugar level. All samples of a given pure substance have the same composition regardless of their origin.
Benedicts solution can be used to test for glucose fructose maltose and other sugars but not for sucrose. Retina REH-ti-nuh The light-sensitive layer of tissue that lines the back of the eye. In lab a student heats a sample of the enzyme lactase to 100C.
20 Words Used To Describe Specific Tastes And Flavours. - The interaction of mass with gravity creates weight. What is the.
A pure substance participates in a chemical reaction to form predictable products. Acidic very sour. The testing strip will change color to purple to indicate glucose is present.
The cell is in Hypotonic Solution. Glucose is a simple sugar with the molecular formula C 6 H 12 O 6Glucose is the most abundant monosaccharide a subcategory of carbohydratesGlucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide using energy from sunlight where it is used to make cellulose in cell walls the most abundant carbohydrate in the world. But in negative control it will stay blue see figure attached for explanation.
Sweet has the molecular formula C 6 H 1 2 O 6. Next the lactase is added to a solution of lactose. B All three water samples would produce hydrogen and oxygen in a 21 mole ratio.
Substance electrical conductivity when solid electrical conductivity when molten melting point solubility in water A does not conduct does not conduct low insoluble B conducts conducts high insoluble C does not conduct does not conduct very high soluble D does not conduct conducts. Examples of monosaccharides in foods are glucose fructose and galactose. The chemical formula of glucose is C6H12O6 so it is classified as an.
The chemical test for glucose is the Benedicts solution test. An atom is the smallest particle of an element that still has all the properties of the element. Glucose from Greek glykys.
It is obtained directly from the diet principally following the hydrolysis of ingested disaccharides and polysaccharides and by synthesis from other substrates in organs such as the liver. Monosaccharides Greekmonos singlesacchar sugar or simple sugars consist of one sugar unit that cannot be further broken down into simpler sugars 1. Monosaccharides are the simplest form of carbohydrates.

5 E Lesson Plan What Are Chloroplasts Photosynthesis How To Plan Lesson

Pin By Joey Chee On Homeschool Essay Writing Academic Writing Writing

Distance Learning Lab Reducing Sugars Distance Learning Learning Lesson

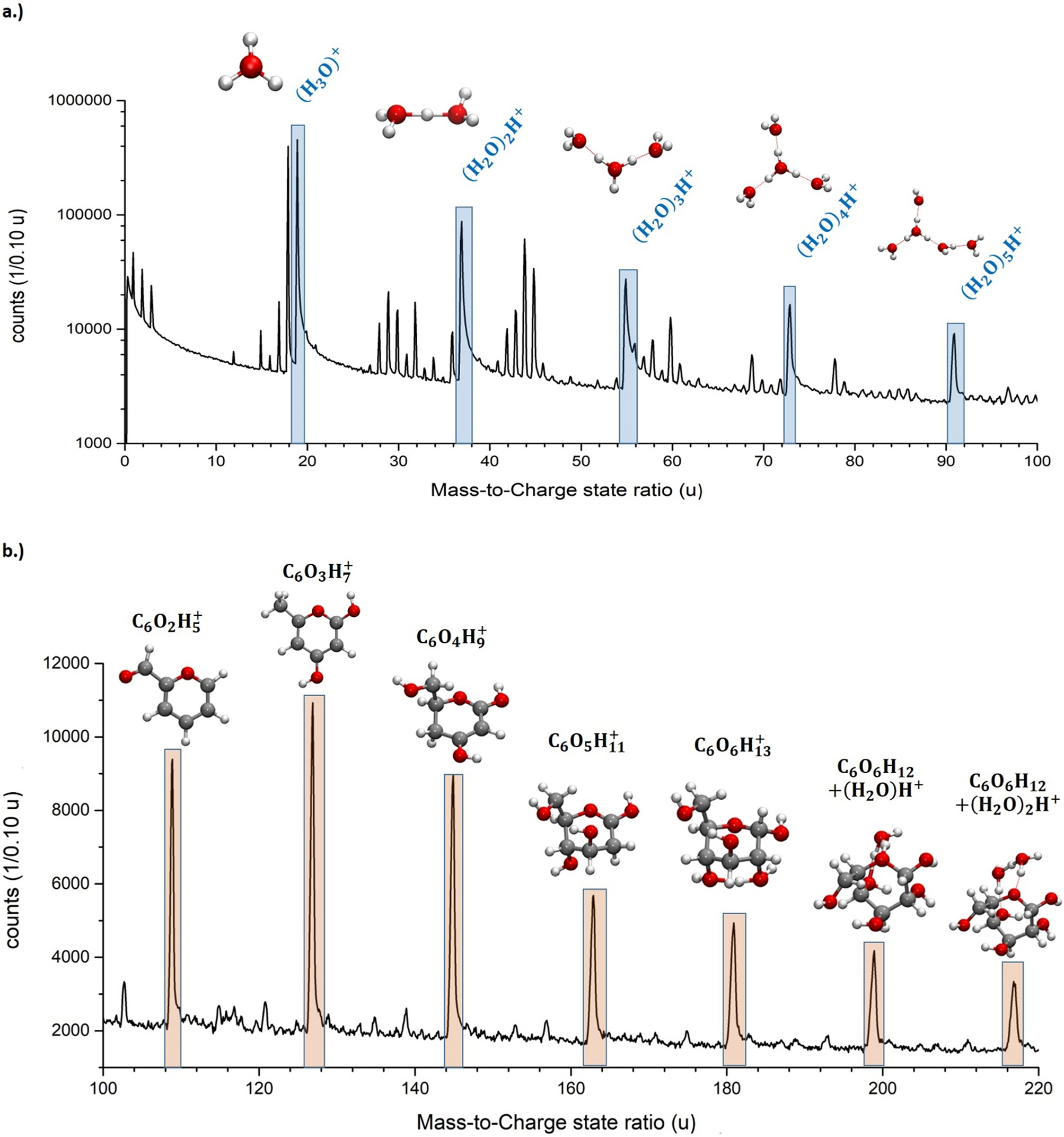
3d Sub Nanometer Analysis Of Glucose In An Aqueous Solution By Cryo Atom Probe Tomography Scientific Reports
Comments
Post a Comment